Many people my age will remember the television show Mission Impossible from the late 1960s. At the very beginning of each episode, the lead character, Jim Phelps (played by actor Peter Graves) and his team of secret agents would receive a new mission from headquarters, and they would have 60 minutes (of TV time) to execute it in the face of all sorts of obstacles. The instructions and background information for the Impossible Mission Force team were always provided in a large brown envelope and a reel-to-reel audio tape player. At the end of the tape, the voice would always say, “This tape will self-destruct in five seconds. Good luck, Jim.” The tape player would suddenly start smoking and you would see the tape warping and melting as it succumbed to the mysterious fuming and heating. It almost seemed that the device was undergoing spontaneous combustion.
If you think about it, self-destruction is the essence of spontaneous combustion, but spontaneous combustion is not limited to items that have outlived their usefulness. In fact, when spontaneous combustion occurs, it almost never happens because someone intended for the material to be incinerated.
Regardless, the nagging question about spontaneous combustion not how, but why would the material survive for years or decades without undergoing self-ignition, and then one day, its surroundings change and its own tendency to undergo self-heating chemistry launches its voyage toward self-ignition and self-destruction? To paraphrase James Carville, “…It’s the environment, stupid.”
The classic scenario for enabling spontaneous combustion is filling a waste basket with linseed oil-soaked rags and waiting 3 to 12 hours. Linseed oil, like most vegetable oils, contains oxygenated hydrocarbon and these constituents continuously undergo exothermic reactions. Thankfully, the reaction rate is so slow that under normal conditions, the (neat) oils naturally dissipate such heat through conduction and convection and the liquid never warms up or approaches thermal runaway.
Conversely, when a film of the same oil is adhering to the woven interstices of cloth rags accumulated into a pile, the self-heating reactions occurring at the center of the pile deposit their heat into a space that is well insulated from the surroundings, and the heat loss is minimal. This allows the temperature to rise locally, which causes the reaction rate to grow exponentially, and eventually thermal runaway is reached. At that point, the limitation on how much longer it will take for the material to begin smoldering and to ultimately ignite is governed by the ability of fresh air to diffuse through the openings in the rags and to provide oxygen to the reacting organic material at the center of the pile.
NFPA cites the following statistics about spontaneous combustion or chemical reaction caused fires: average of 14,070 fires per year between 2005 and 2009, including 3,200 structure fires, 1,150 vehicle fires, 5,250 outside non-trash fires, and 4,460 outside trash or rubbish fires. The following are known to be capable of spontaneous combustion: Activated carbon, Oily cotton, Paper, Seed cake, Celluloid scrap, Linen stacks, Log piles, Coal piles, Haystacks, Compost piles.
Agricultural raw materials and food or feed products are very likely to undergo spontaneous combustion – especially if they are being heated in an oven or dryer. NFPA 61 states: “Spontaneous ignition is a primary cause of dryer fires and explosions. The requisites of this phenomenon are a heated surface or a hot airstream, a layer of product exposed to this heat, and time.” The four factors that make spontaneous ignition more likely are: (a) size of pile and effectiveness of self-insulation; (b) temperature of surroundings; (c) ability of oxygen to diffuse to the reaction zone; (d) sufficient time for self-heating reactions to accelerate.
Unfortunately, safe handling of linseed oil-soaked rags is not entirely intuitive, and spontaneously ignited fires continue to occur via many different scenarios. Warning labels on cans of linseed oil state: “USE EXTREME CAUTION. Immediately after use and before disposal or storage, you MUST (1) hand-wash rags thoroughly with water and detergent outside in a bucket and rinse. Repeat washing and rinsing until you have removed all oil from all cloths, rags, paper, … and any other materials contacted during use or because of an accidental spill. (2) spread all rinsed materials outside to dry by flattening them out to their full size in an airy spot for at least 24 hours until completely dry.”
This author has investigated fires where spontaneous combustion was the only likely ignition source and others where allegations of spontaneous combustion were proven incorrect upon further investigation.
The purpose of “Investigation Anecdotes” is to inform our readers about the intriguing field of engineering investigations. We hope you are instructed by this content, and we encourage you to contact us if you seek additional information.


Monthly Archives: November 2016
Boiler Purge Causes Explosion
During a recent explosion investigation, this author discovered a new failure mode that is not sufficiently addressed in NFPA’s trio of industrial heating equipment standards (NFPA 85, NFPA 86, and NFPA 87) that cover Boilers, Ovens, and Fluid Heaters, respectively. The failure mode occurs only in heating systems equipped with natural gas burners and flue gas recirculation (FGR) for control of NOx emissions. The investigation where the failure mode manifested itself happened to be concerned with a boiler explosion, but ovens, furnaces, and fluid heaters are equally capable of experiencing the same problem, if certain factors are in play.
The schematic below identifies the primary equipment that plays a role in the incident scenario. In addition to the boiler, burner, blower and natural gas source, there are two flow valves (FV-001 and FV-002) that control the amount of FGR blended with fresh air that enters the burner. On smaller boilers, FV-001 is set manually during commissioning to approximately 50% open and rarely changed, whereas FV-002 is typically an automatic valve with two discrete positions – closed (no recirculation) and normal (standard recirculation).
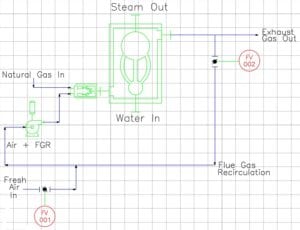
NFPA burner safety requirements require a pre-ignition purge at the beginning of each burner startup to help ensure the combustion chamber is free of residual fuel gas or any other combustible vapor. NFPA burner standards have included a purge requirement for at least 50 years and such requirements have reduced the rate of explosions significantly.
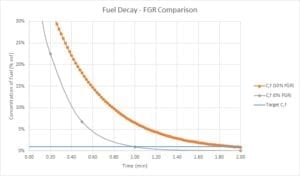
The goal of the purge cycle is for approximately 4 volumes of fresh air to be admitted into the combustion chamber to drive out any unwanted combustible gas or vapor. For example, if the combustion chamber has a volume (𝑉 = 100 ft3) and the blower is delivering a flow rate Vdot = 400 acfm the purge time should be 𝑡 =1.0 min. This amount of purge is almost always conservative enough to ensure combustible vapors are diluted to a nonflammable concentration in the firebox. The very first volume of fresh air purge in theory is enough to remove the combustible vapors if a plug flow model is assumed for the air flow inside the chamber. The requirement for 4 purge volumes arises from the fact that the plug flow model isn’t conservative enough if plug flow behavior is not achieved. Hence, a perfectly-stirred vessel model is used instead. The decay of fuel concentration in the firebox is exponential with time, and 4 volumes of purge air will take a 50% fuel concentration down to 1%.
However, if the purge air isn’t comprised of pure air, but rather a mixture of “flue” gas with a high concentration of unburned fuel from the prior unsuccessful burner ignition attempt, the purging process is much slower. The figure below shows the difference in decay rates between the normal case, where the purge air is 100% air, and the compromised case, where the purge air comprises 50% FGR (with residual fuel) and 50% fresh air. When purge is carried out with contaminated air, the number of purge volumes required is 8, not 4.
For the boiler explosion case described above, this author found that FV02 had been unplugged from its power source and the damper was stuck in a partially open condition. After 3 unsuccessful ignition trials in rapid succession, the spark igniter set off an internal deflagration that damaged the vessel walls such that a complete replacement of the boiler was required.